Ultralight
Compliance automation for medtech innovators
Ultralight helps medical device manufacturers navigate the complex regulatory landscape from design through to FDA approval. We served as their design partner for over two years, helping them go to market and expand their business once they were established.
-1744950506.jpg)
NBS x Ultralight
Designing the all-in-one medtech product & compliance hub
We partnered with Ultralight on day zero, working directly with CEO Monik Sheth and CTO Shiv Ghai to shape how Ultralight would create a product which served its mission: unlocking more innovation in medtech by simplifying and streamlining the incredibly complex compliance and FDA approval process.
Understanding FDA Approvals
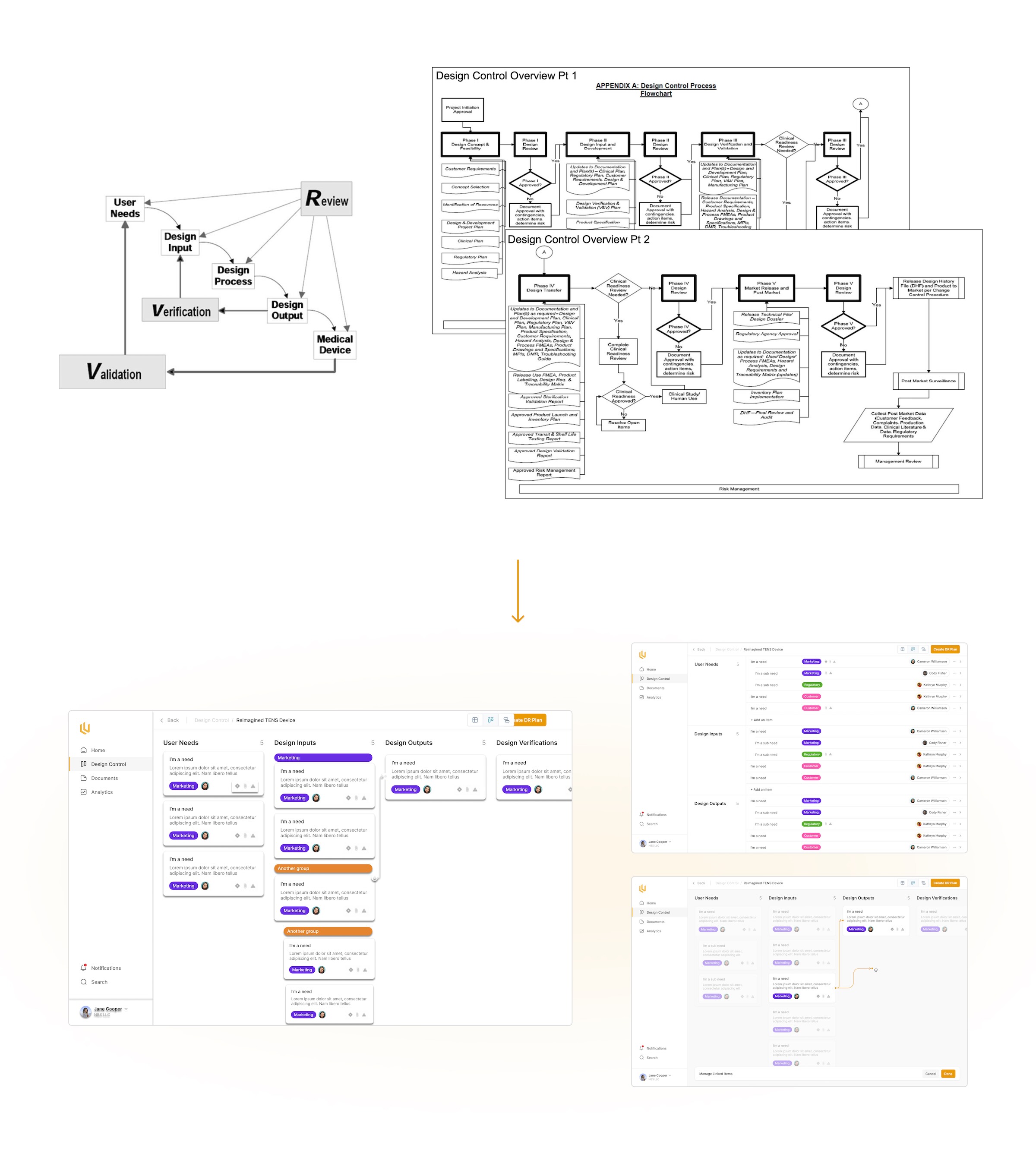
Right at the beginning of our partnership we began deconstructing how medical software and hardware devices get approved. Over a number of weeks we dove into the FDA's documentation on this, exploring various ways we could contextualize this in the product.
This quickly began to turn into some rough, preliminary mockups of what would eventually become Ultralight's 'Design Control' feature
Over 3 years we helped shape Ultralight's product, right up to its acquisition in 2025
We served as Ultralight's product and design team for 3 years, complimenting their in-house engineering team. We were proud to work with the Ultralight team right until their acquisition by Greenlight Guru in 2025.
Design Control
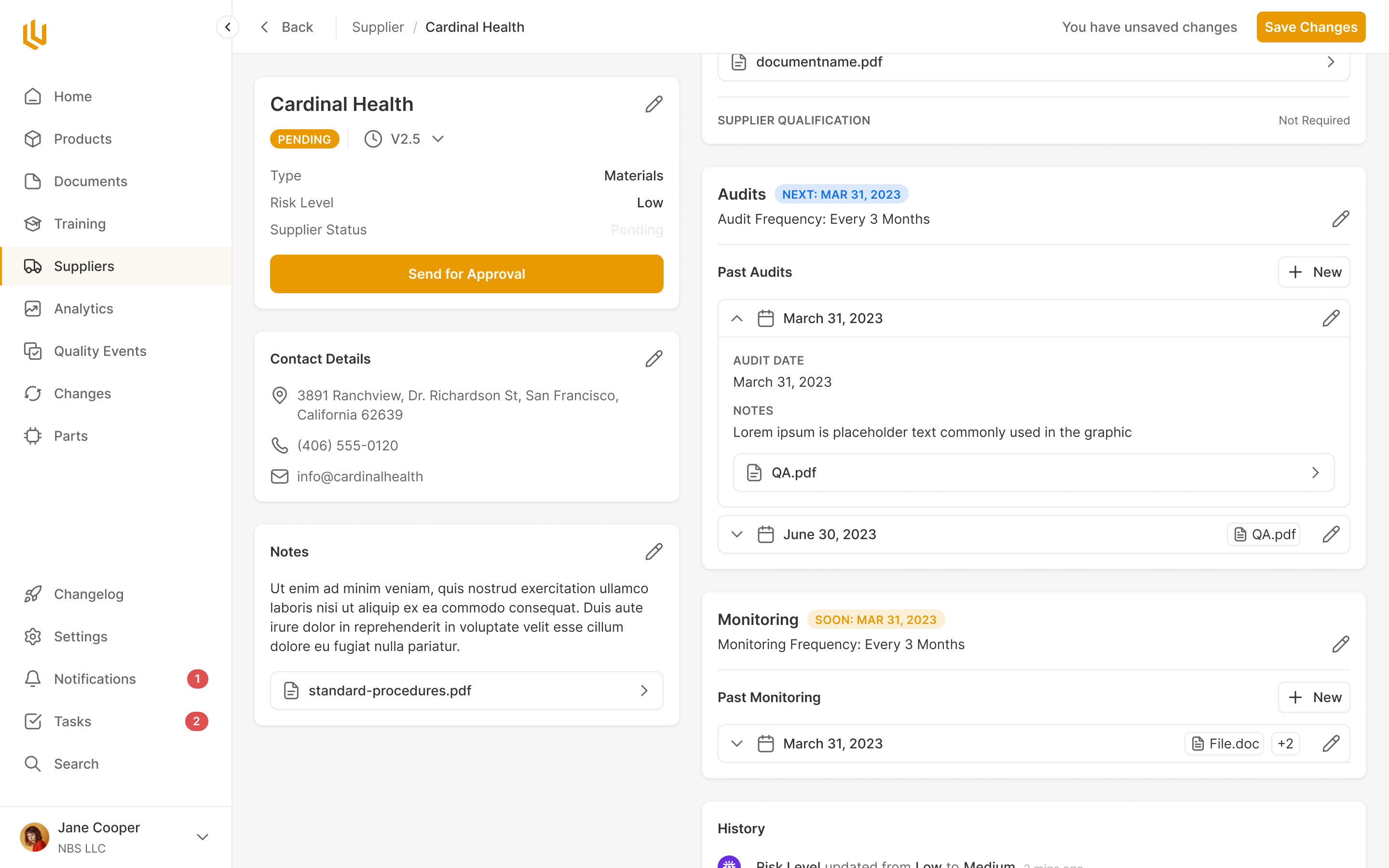
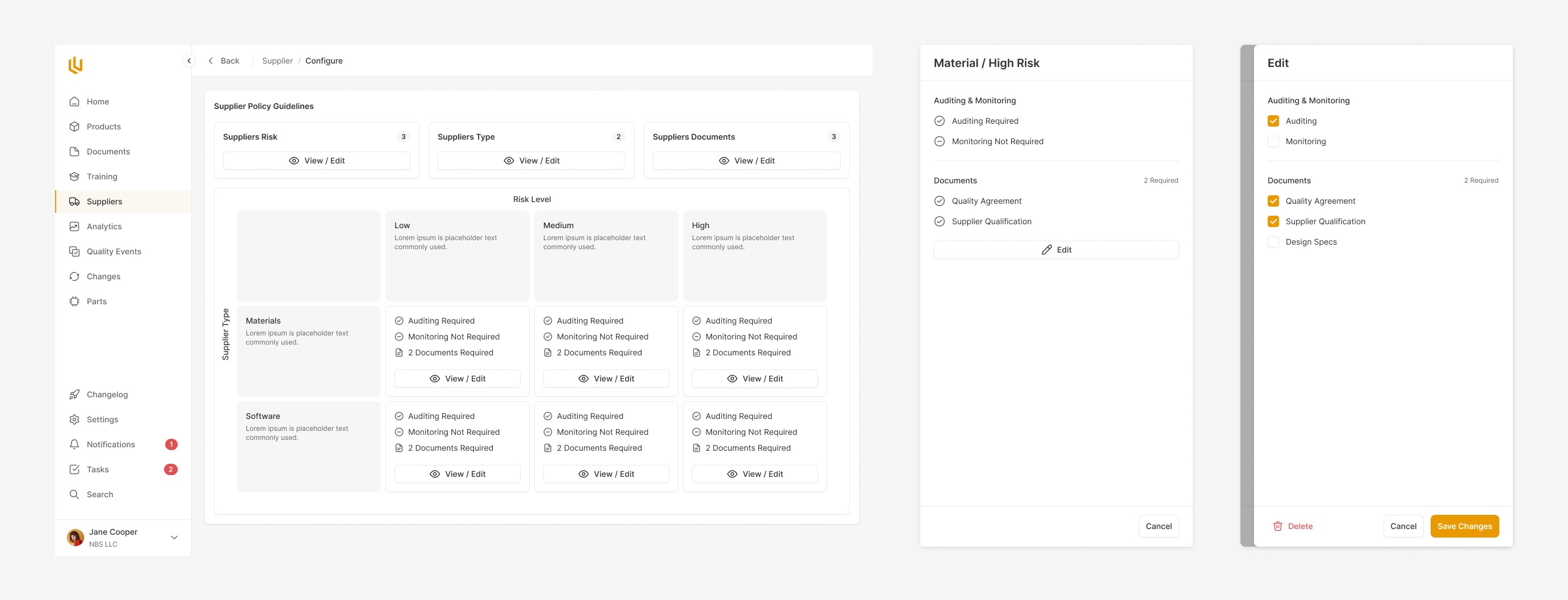
The Design Control module within Ultralight is the core of where design decisions, requirements and review take place. From the very conception of a med device, the every decision made has to be documented. The Design Control allows engineers and managers to clearly note decisions throughout the product design process, making future approvals a breeze.
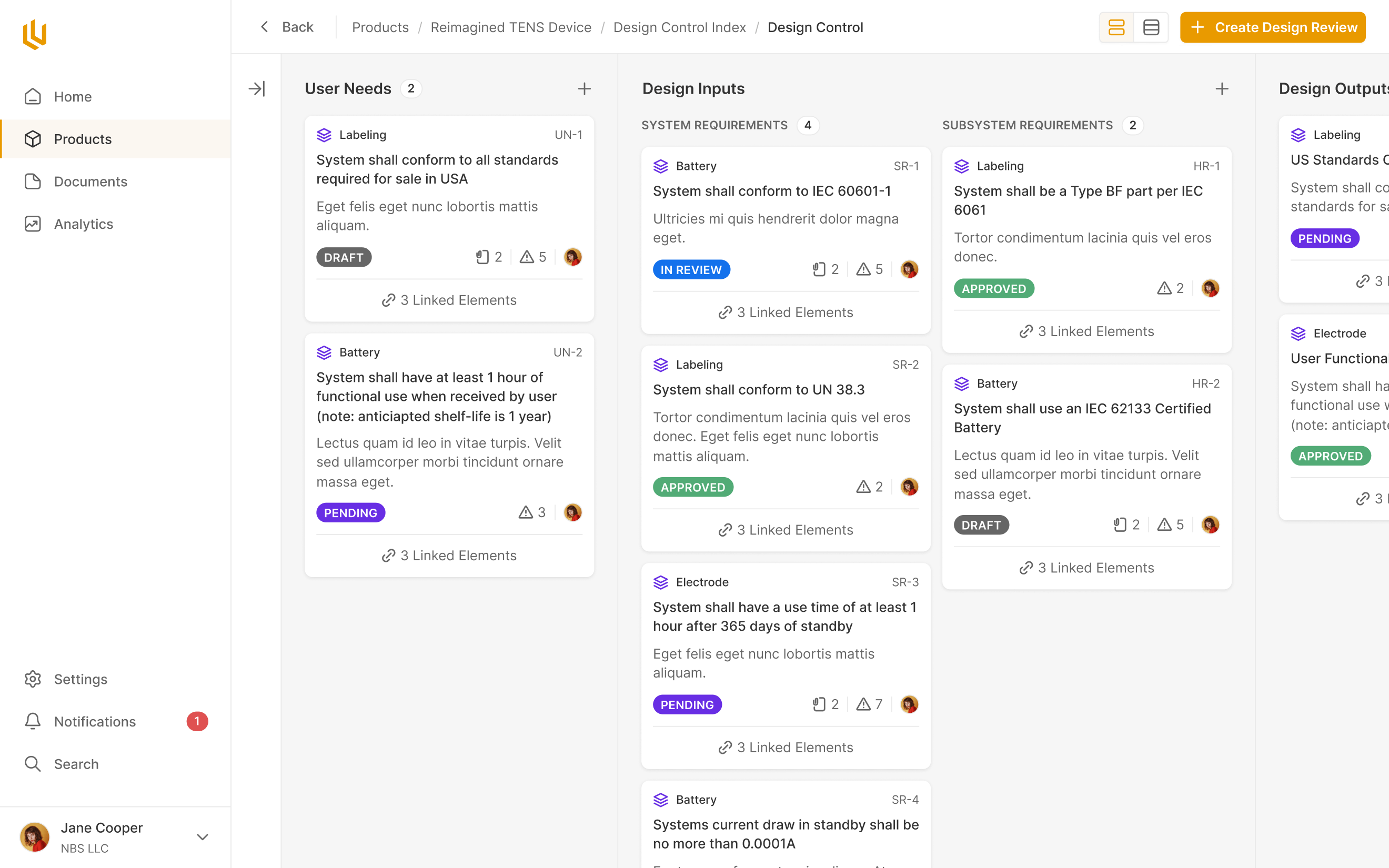
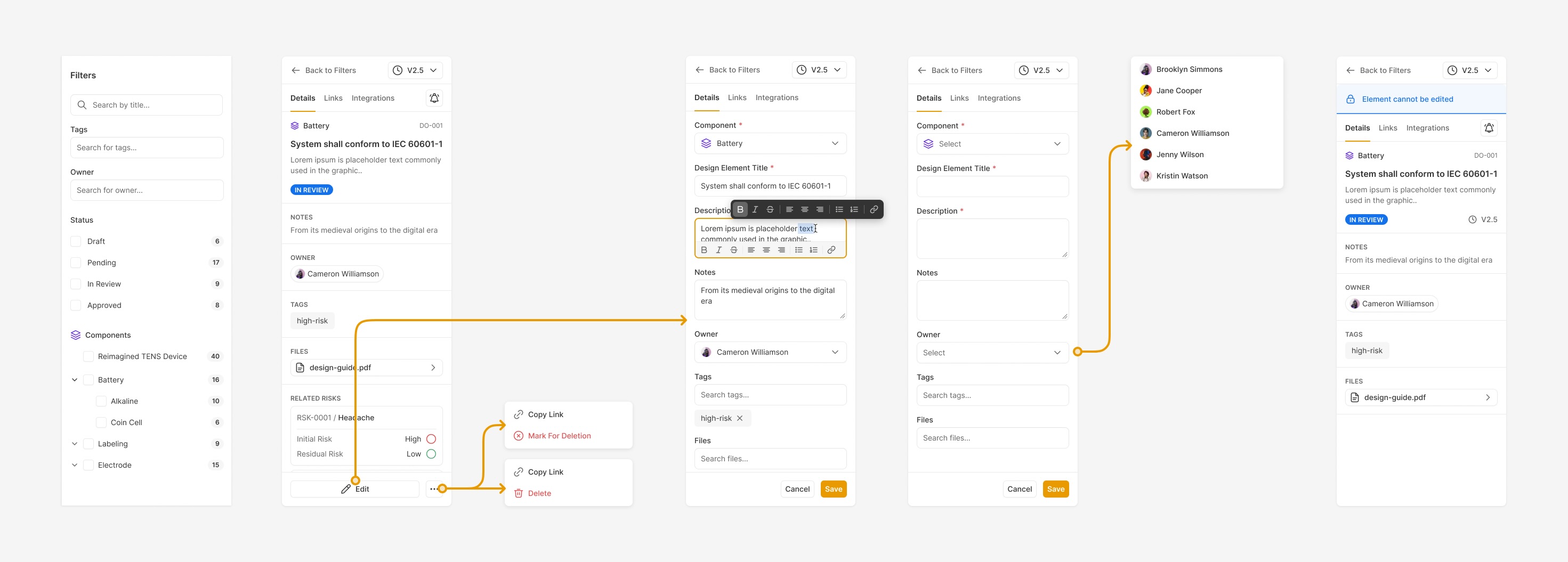
The multi-functional tool panel we created for Ultralight's Design Control module did a lot of heavy lifting in allowing users to edit a lot of information in a compact environment
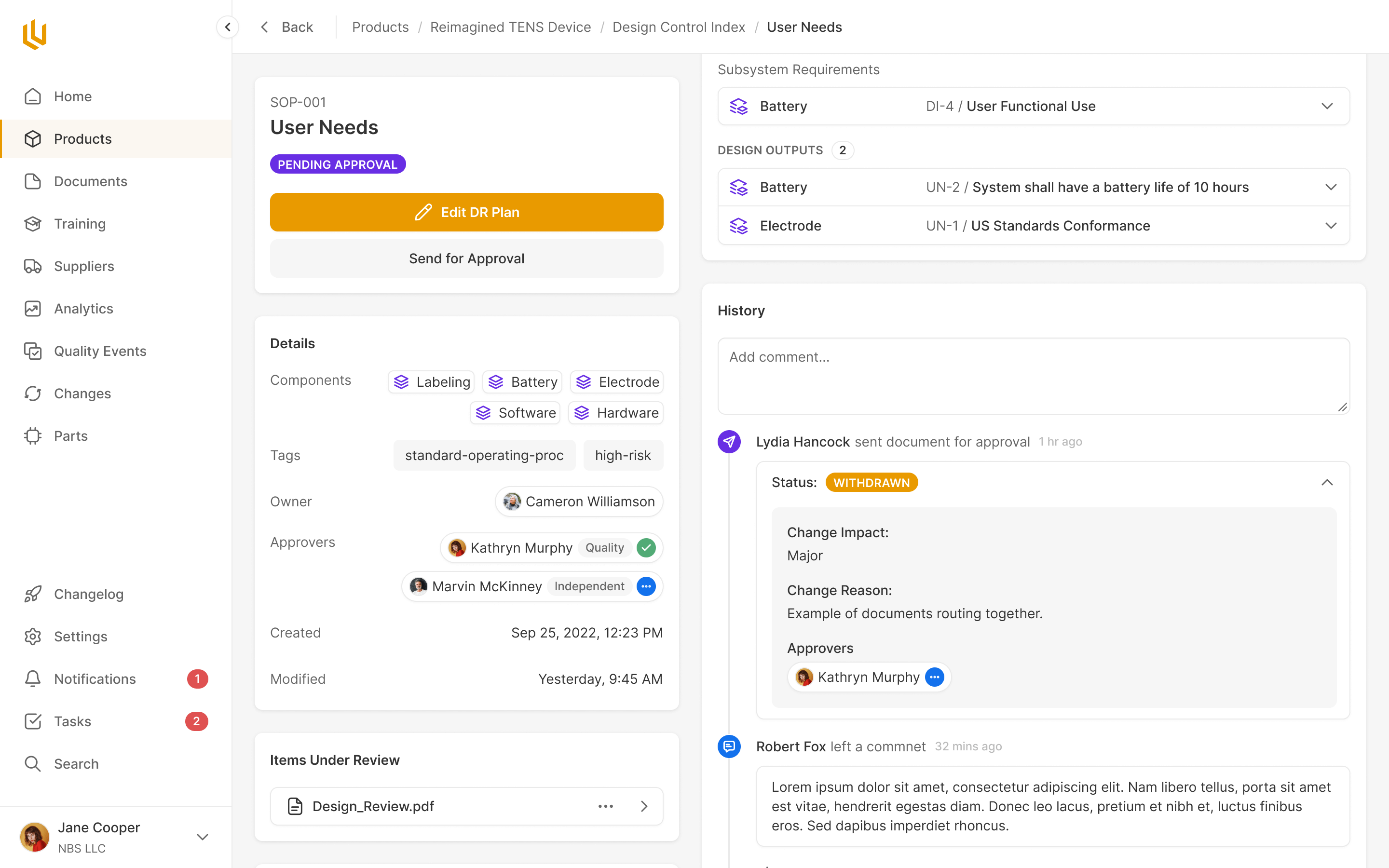
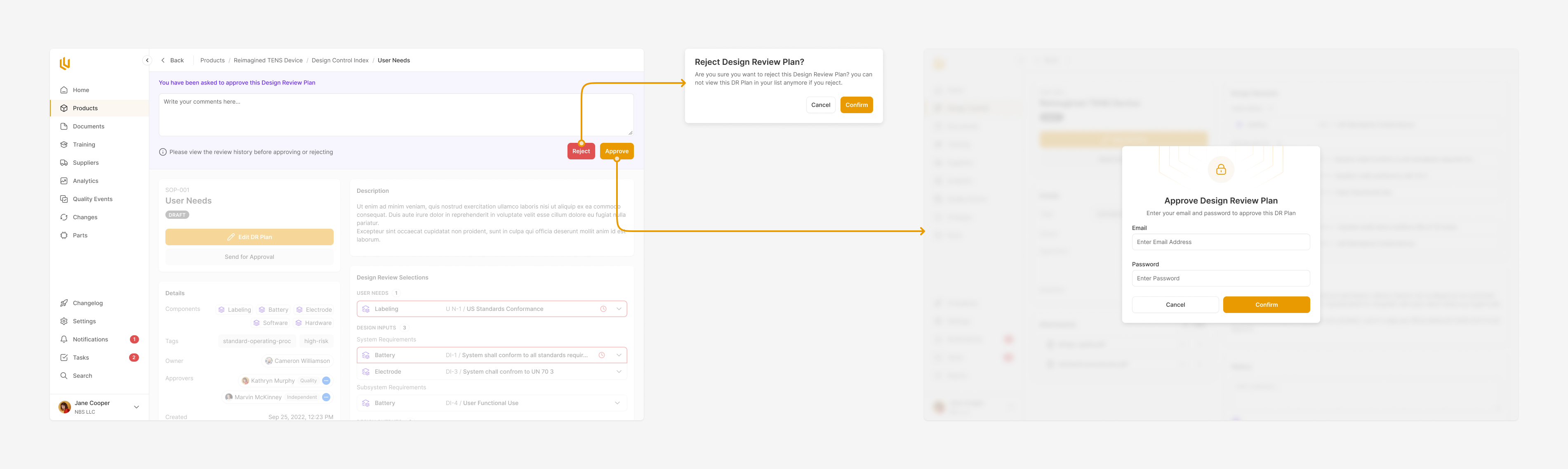
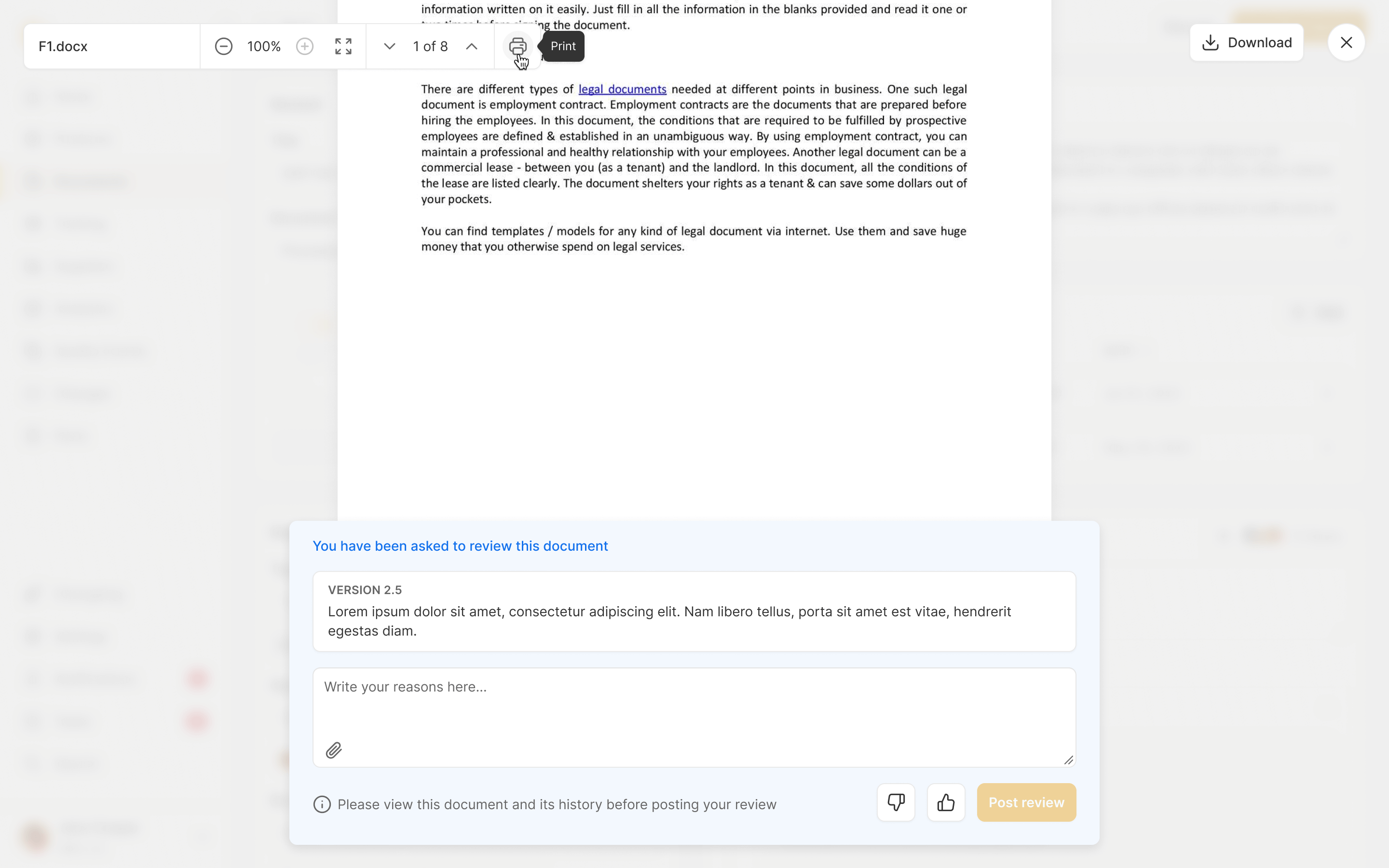
Design Review
Every core component to a product has to go through a clear approval process. Ultralight streamlines this by pulling together all the information for reviewers to see, and automatically reminding the appropriate team members, handling rejections and approvals seamlessly.
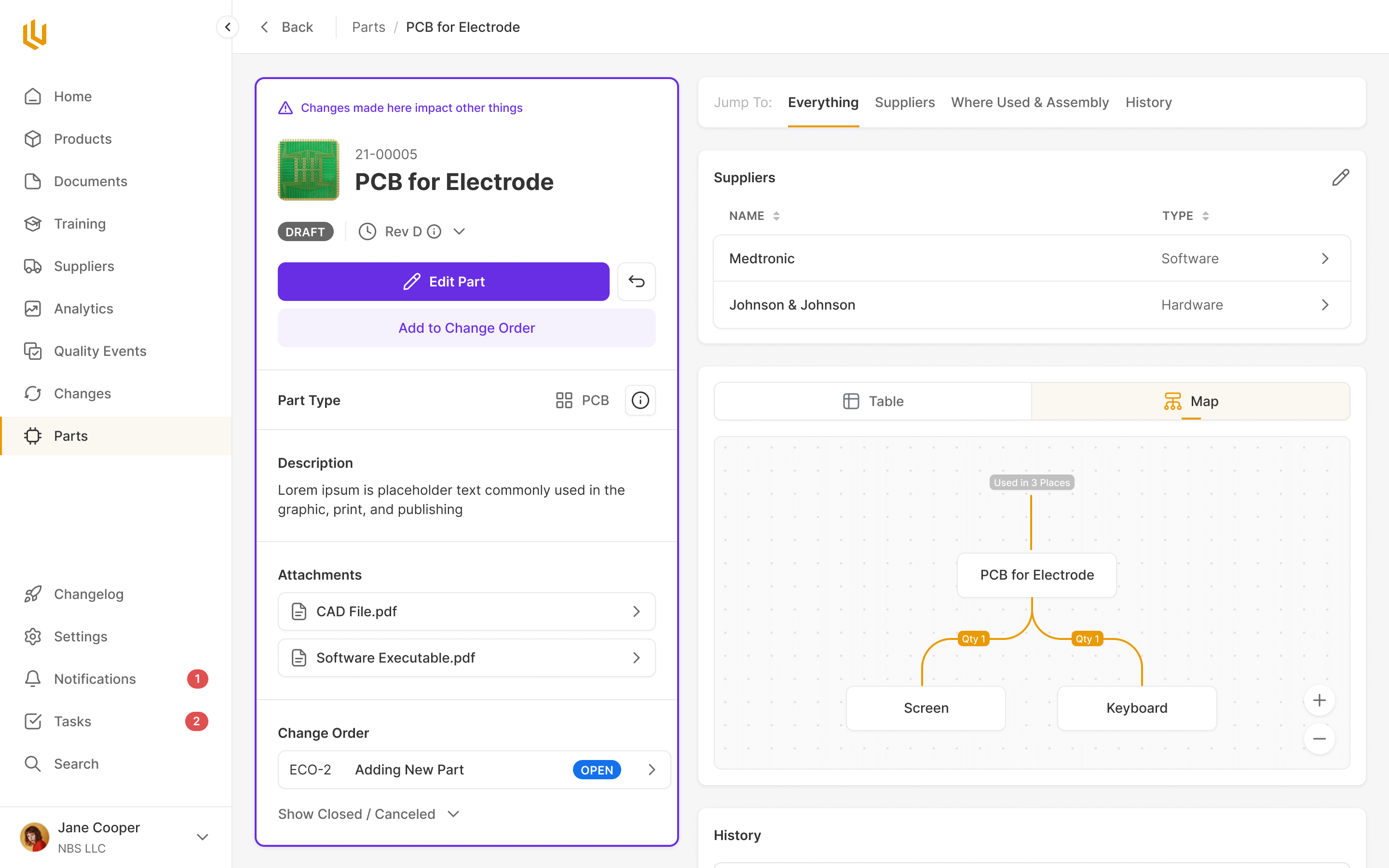
Parts
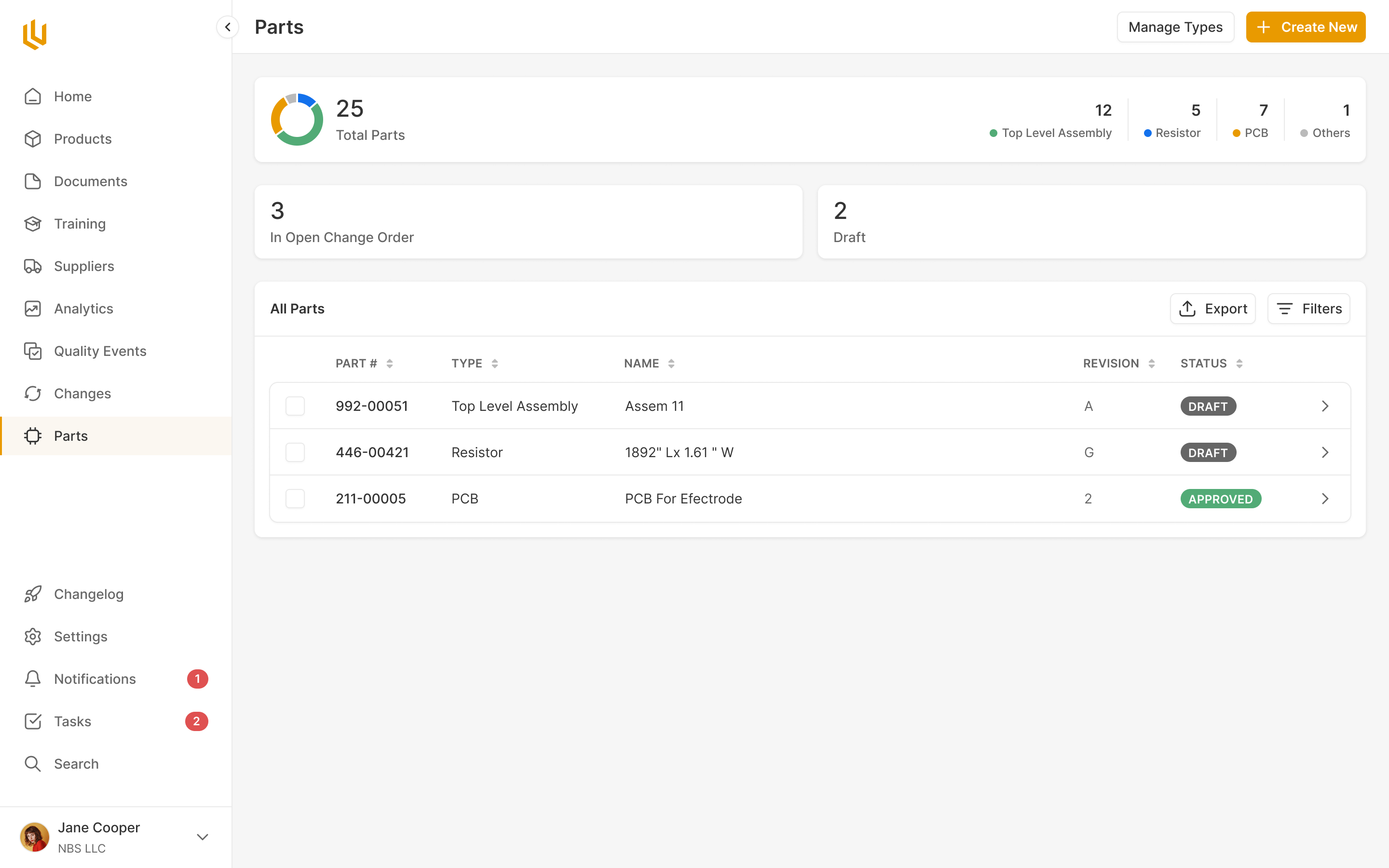
Simple parts supply chain documentation
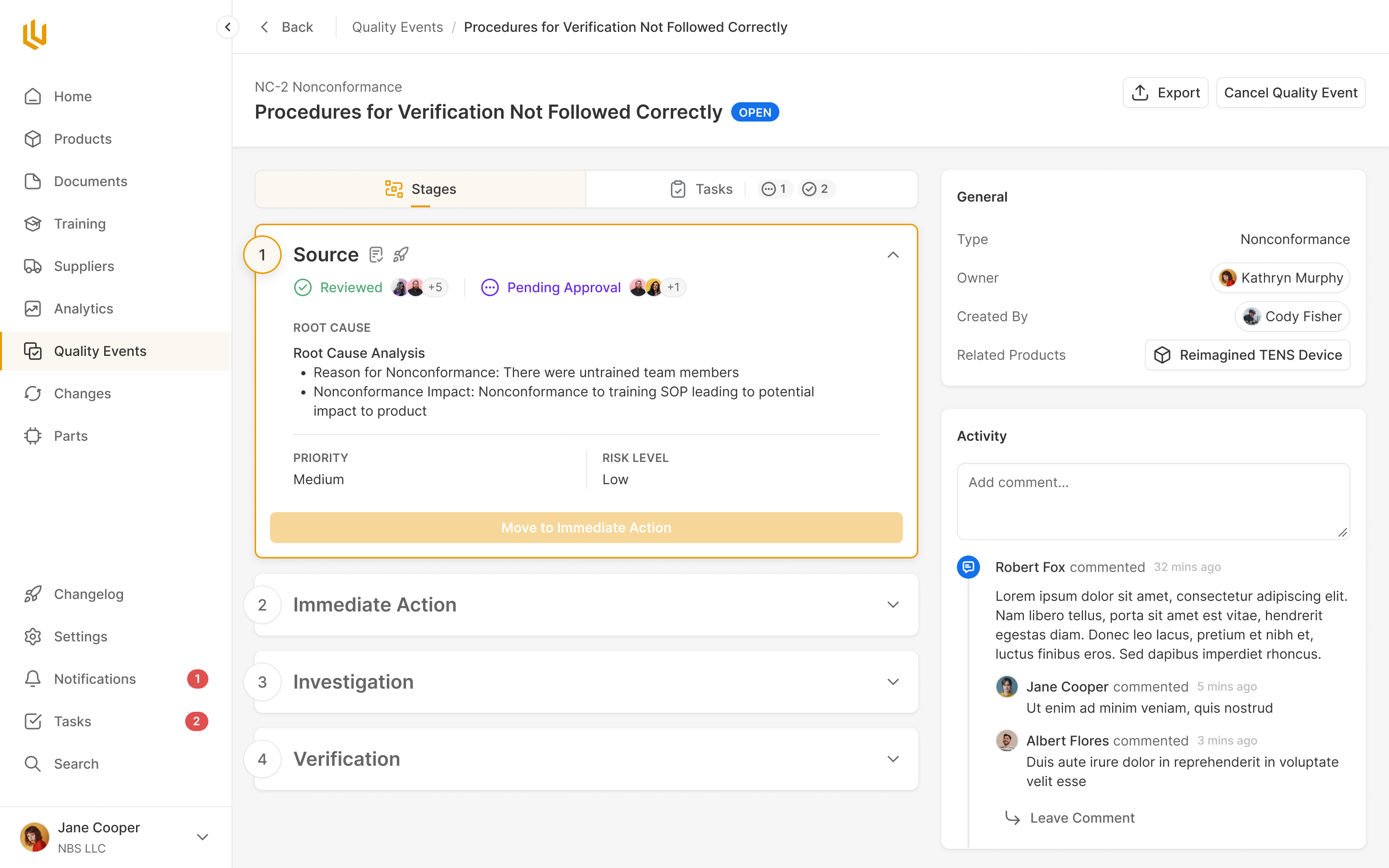
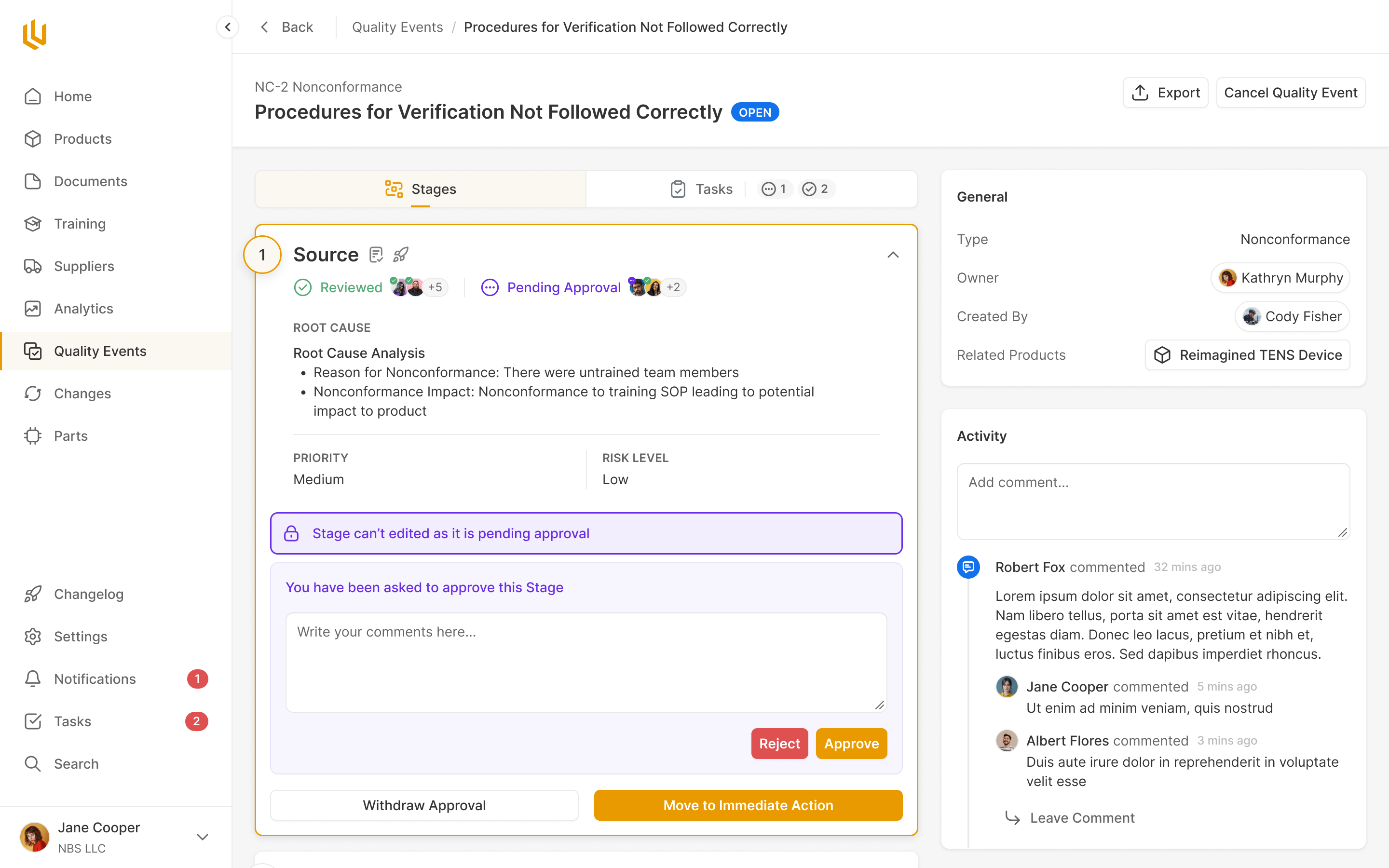
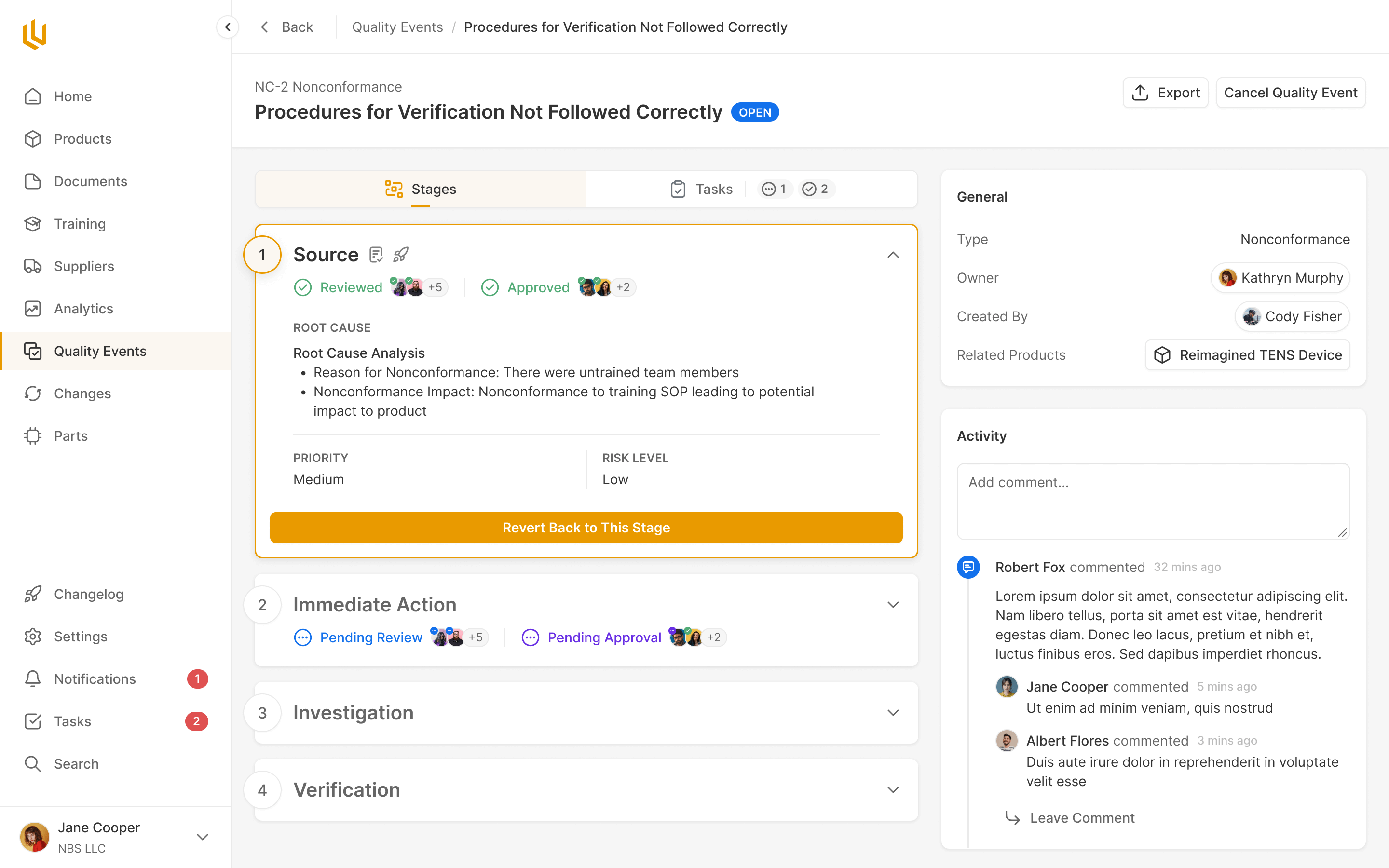
Quality Events
Critical decision or change events are handled within Ultralight, with all compliance steps pre-defined for the team to work through
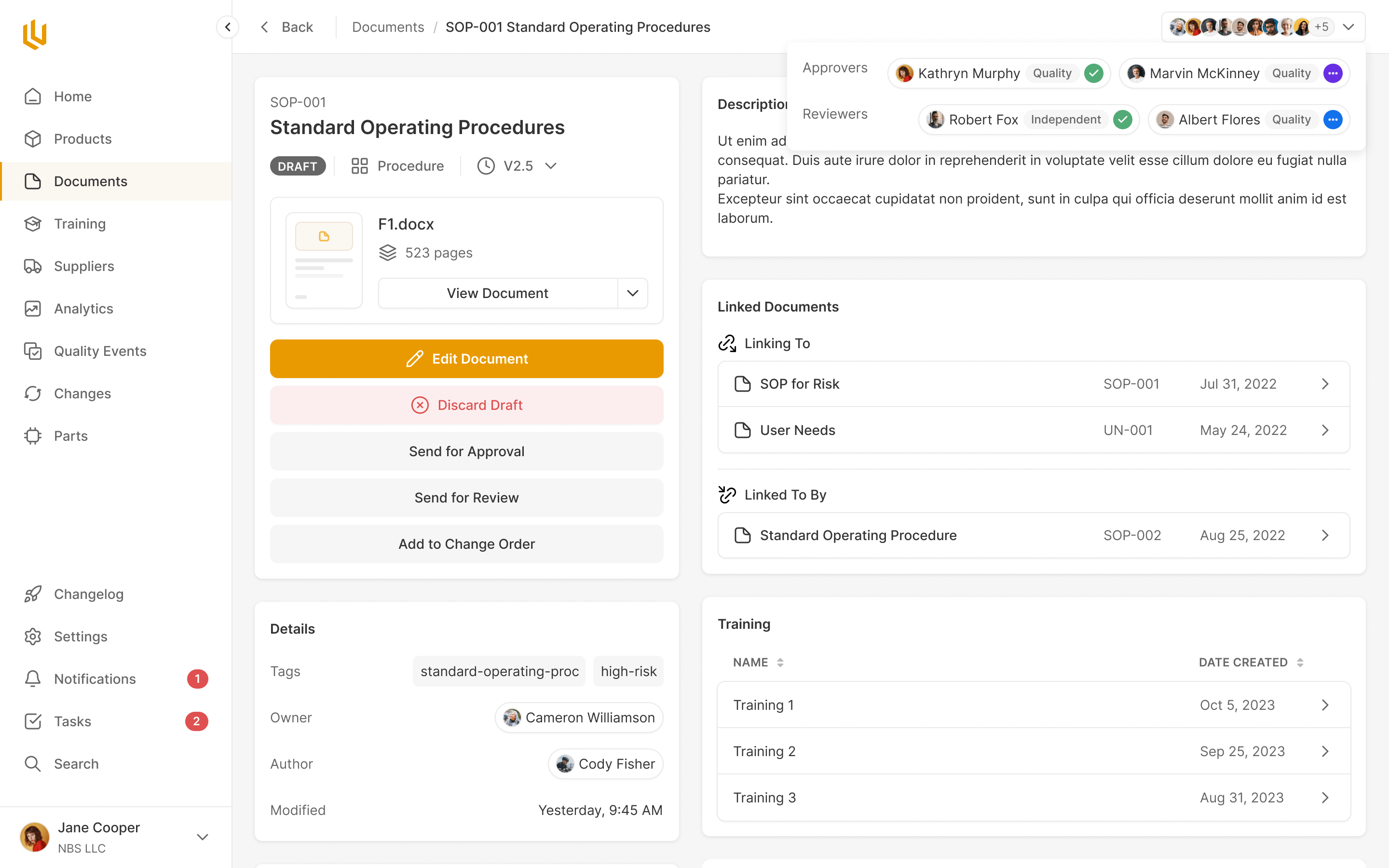
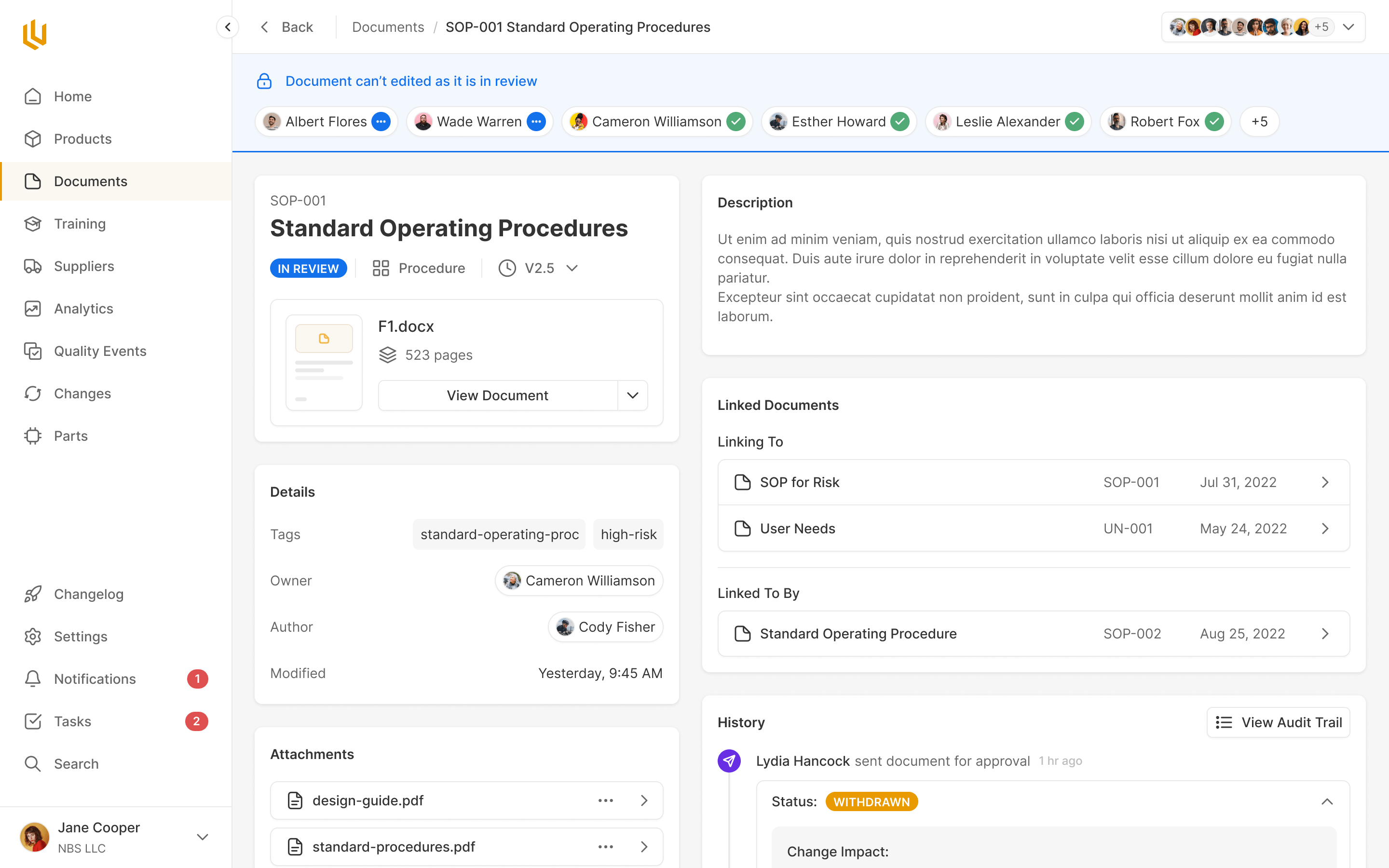
Documents
Company and product documentation needs approval under FDA regulations, the same as design specifications. Ultralight handles document storage and approval using the same process users are already familiar with.
Supplier Management
Teams using Ultralight can ensure ongoing compliance of suppliers right inside the platform